Press release -
Efficacy of Opdivo (Nivolumab) in Cancer of Unknown Primary Cases Confirmed - Kindai University
- Efficacy of molecular target drug Nivolumab verified in investigator-initiated clinical trial for use with CUP by hospital team from Kindai University’s Faculty of Medicine
- Reductions by more than half in tumor size in over 20% of CUP patients observed
- Nivolumab expected to become standard treatment for CUP cases with no standard treatment
Osaka-Sayama, Osaka, Japan. May 31, 2020
The research team centered around Prof. Hayashi of Kindai University (Osaka-Sayama, Osaka, Japan) Faculty of Medicine (Department of Medical Oncology) has verified, for the first time in the world, demonstrated efficacy of the use of the molecular targeting drug, Nivolumab (product name: Opdivo) on patients with cancer of unknown primary (CUP) in a phase 2 trial (investigator-initiated clinical trial*1).
May 31st, 2019 (Sun) at 6:30 AM Japan time, at the "Special Clinical Science Symposium", held by the world's largest academic oncology society, American Society of Clinical Oncology – where highly anticipated scientific findings are released – Assistant Professor Tanizaki of Kindai University's Faculty of Medicine, Medical Oncology Dept. will publicly release these results online.
Background of Research
In the case of regular cancers, whether it be lung cancer or stomach cancer, the primary lesion is in these organs, however in CUP cases the primary lesion's origin has not been able to be located, and metastasis to the likes of the lymph nodes and liver is found, and the cancer spreads throughout the body. CUP accounts for 2-5% of cancer cases, and generally has a poor prognosis, with a 1-year survival rate from diagnosis for approximately 50% of cases. Over half of patients have metastasis and the presence of cancers in multiple organs already at the time of diagnosis. Exceedingly poor prognosis is characteristic of CUP; the median survival rate is approximately 6-9 months, and the 5-year survival rate is 2-6%. Further, as patients present with various symptoms and diagnosis is difficult, compared to other cancer types, development of treatments has not occurred.
Although immune checkpoint inhibitors*2, such as Nivolumab, are a part of standard treatment for a number of types of cancers, there are only a few reports regarding the effectiveness of immune checkpoint inhibitors for CUP. Kandai University's Faculty of Medicine, Oncology Department analysed the results of immune profiling of CUP, and from that reported in the below paper, in 2019, that it anticipated immune checkpoint inhibitors may be effective against CUP.
Haratani K, et.al, J Immunother Cancer. 2019 Sep 13;7(1):251, Clinical and immune profiling for cancer of unknown primary site.
Overview of Research
With that background in mind, Kindai University Hospital Clinical Research Center and Department of Medical Oncology, Kindai University Faculty of Medicine, lead an investigator-initiated phase 2 clinical trial on the efficacy of Nivolumab (Opdivo) with CUP patients (NivoCUP trial), conducted at 10 facilities around Japan. Results showed a 22% observed response rate*3 for CUP patients who had previously received chemotherapy treatment; and, as 32% of patients experienced successful suppression of cancer for over six months due to treatments, Nivolumab (Opdivo) is anticipated to become the standard treatment for CUP. Lastly, this research was made possible due to the funding and fee-less trials of the medicine by Ono Pharmaceutical Co Ltd.
Academic Conference Presentation
American Society of Clinical Oncology (ASCO): Special Clinical Science Symposium: Redefining
Cancer of Unknown Primary: Is Genomics the Answer?
Saturday, May 30, 2020: 3:30 PM-4:30 PM(CDT)
(Sunday, May 31, 2020: 5:30 AM-6:30 AM (JST))
Title:NivoCUP : An open label Phase 2 study on the efficacy of nivolumab in patients with cancer of unkwnon primary (CUP)
Presenter:Junko Tanizaki, Assistant Professor at Department of Medical Oncology, Kindai University Faculty of Medicine. Department of Medical Oncology, Kishiwada Municipal Hospital.
Co-researchers: Junko Tanizaki1,2, Kan Yonemori3, Kohei Akiyoshi4, Hironobu Minami5, Hiroki Ueda6, Yuichi Takiguchi7, Chihiro Kondo8, Yoshihiko Segawa9, Makoto Takahashi10, Yasuo Iwamoto11, Yasuhiro Kidera12, Kazuya Fukuoka12, Akihiko Ito13, Yasutaka Chiba12, Kazuto Nishio14, Kazuhiko Nakagawa1.
Corresponding author: Hidetoshi Hayashi1, Lecturer, Department of Medical Oncology, Kindai University Faculty of Medicine
Kindai University Faculty of Medicine 1Department of Medical Oncology, 13Department of Pathology, 14 Department of Genome Biology/
2Department of Medical Oncology, Kishiwada Municipal Hospital/ 3Department of Breast and Medical Oncology, National Cancer Center Hospital/ 4Clinical Oncology Center, Osaka City General Hospital/ 5 Medical Oncology and Hematology, Kobe University Hospital/ 6 Department of Pulmonary Medicine and Oncology, Wakayama Medical University Hospital/ 7Department of Medical Oncology, Chiba University Hospital/ 8Department of Medical Oncology, Toranomon Hospital/ 9 Department of Medical Oncology, Saitama Medical University International Medical Center/10Department of Medical Oncology, Tohoku University Hospital/ 11Department of Medical Oncology, Hiroshima City Hiroshima Citizens Hospital/12Clinical Research Center, Kinki University Hospital
Details of Research
This research was an investigator-initiated clinical trial, with 10 facilities from all over Japan participating, led by Senior Administrator Nakagawa, Prof. Hayashi and Asst. Prof. Tanizaki of the Medical Oncology Department of Kindai University Faculty of Medicine, and Prof. Fukuoka (Director), Asso. Prof. Chiba (responsible for statistical analysis) and Pharmacist Kidera (Assistant Department Director) of Kindai University Hospital's Clinical Research Center.
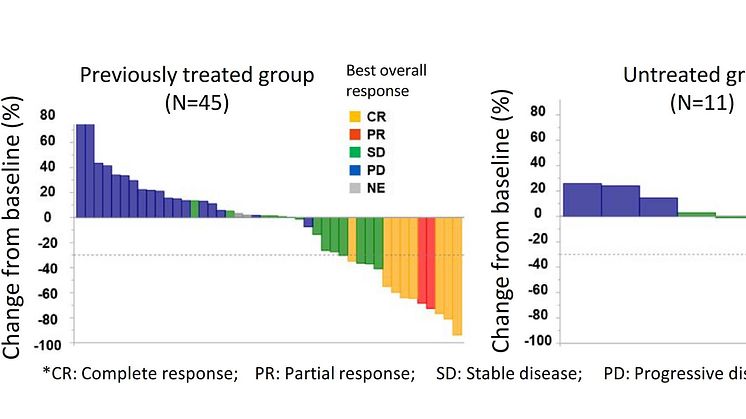
A total of 56 people participated in the study, including 45 patients who had a history of anticancer drug treatment (previously treated group) and 11 patients who had no history of anticancer drug treatment (untreated group) for CUP.
The primary evaluation item for this study was achieved – with the observed response rate of the previously treated group coming in at 22.2% (95% conf. int.: 11.2-37.1%). The median value of the progression-free survival period (period from start of treatment to worsening of cancer) for the previously treated group was 4.0 months (95% conf. int.: 1.9-5.8 months). At 6 months the progression-free survival proportion was 32%, and median survival period was 15.9 months (95% conf. int.: 8.4-21.5 months). Observed response rate in the untreated group was 18.2% (95% conf. int.: 2.3-51.8%), the median value of the progression-free survival was 2.8 months (95% conf. int.: 1.1-6.5 months), and at six months, the progression-free survival proportion was 27%, and the median survival period was unattained (95% conf. int.: 2.6 months; unachieved). Even when compared with previously reported treatment results of chemotherapy, the treatment results were observed to be effective.
In addition, in a world first, this study showed, as evaluated by Prof. Ito of Kindai University Faculty of Medicine (Department of Pathology), that in cases of CUP patients with high levels of PD-L1 expression*4 in tumor cells, the curative effect was high, and demonstrated Nivolumab's therapeutic value (observed response rate, progression-free period, survival period).
The study showed a 12.4-month response period (95% confidence interval: 2.8 months-not reached) for Nivolumab in the previously treated group. These results were better than the most commonly used combination therapy in clinical practice - platinum and taxane agents – which have a reported median response time of approx. 4 to 7 months. The greatest clinical charateristic of Nivolumab is the persisting efficacy over long-term periods, which prolongs life, but the fact this efficacy is expected to potentially also apply to cases of CUP is an essential point.
This is the world's first investigator-initiated clinical trial to report favourable results for CUP cases, and these results are expected to influence Nivolumab as becoming the standard treatment for CUP cases.
Glossary of Terms
*1 Investigator-initiated clinical trial
Unlike clinical trials run by enterprise-level organizations, planning, designing, and management of the trial is run by physicians.
*2 Immune checkpoint inhibitor
Immune checkpoint inhibitors exhibit antitumor effects by inhibiting the brakes that are used for avoiding immune cells' attack on tumors and enhancing the immune cells' attack.
*3 Observed response rate
The proportion of cases that experience at least about half or more reduction in size of tumor.
*4 PD-L1 expression
Nivolumab is a monoclonal antibody that blocks the binding of PD-1 and PD-L1. It has been proven that the expression of PD-L1 in tumors of various types of cancers is an indicator of the therapeutic efficacy of anti-PD-1 and anti-PD-L1 antibodies, including Nivolumab.
Researchers (Kindai University Faculty of Medicine and Kindai University Hospital Team)
-Department of Medical Oncology, Kindai University Faculty of Medicine
Kazuhiko Nakagawa, M.D.,Ph.D. (Senior Administrator)
Hidetoshi Hayashi, M.D.,Ph.D. (Lecturer)
Junko Tanizaki, M.D.,Ph.D. (Assistant Professor)
-Department of Genome Biology, Kindai University Faculty of Medicine,
Kazuto Nishio, M.D.,Ph.D. (Senior Administrator)
-Department of Pathology, Kindai University Faculty of Medicine,
Akihiko Ito, M.D.,Ph.D. (Senior Administrator)
-Clinical Research Center, Kinki University Hospital
Kazuya Fukuoka, M.D.,Ph.D (Director)
Yasutaka Chiba, Ph.D (Assistant Professor)
Yasuhiro Kidera, Ph.D (Pharmacist)
For media enquiries, please contact koho@kindai.ac.jp , Public Relations Department, Kindai University.
