Press release -
Efficacy of Esophageal Cancer Postoperative Adjuvant Treatment, Peptide Vaccine Treatment, Elucidated 5-year Survival Rate Approx. Doubled for Esophageal Cancer Patients - Kindai University
- Kindai University’s Faculty of Medicine has, for a world first, shown the efficacy of cancer-specific vaccine therapy as a postoperative adjuvant therapy in a phase 2 clinical trial
- The administration of cancer-specific vaccine therapy approximately doubled the 5-year survival rate of advanced esophageal cancer patients.
- The vaccine therapy, that amplifies CTL*2, is expected to contribute to the validation of peptide vaccine therapy, a world first.
Osakasayama, Osaka, Japan. August 31st, 2020
The research team led by Prof. Yasuda of the Dept. of Surgery (Division of Esophago - Gastric Surgery), at Kindai University’s Faculty of Medicine (Osakasayama City, Osaka), has shown for the first time in the world, that the administration of peptide vaccine treatment*1 conducted post-surgery approx. doubles the survival rates, compared to conventional treatment of esophageal squamous cell cancer patients that had confirmed lymph node metastasis with poor prognosis - a cancer that accounts for 95% of esophageal cancer patients in the Japanese population. The research paper will be available online in the world leading journal in the field of surgery, “Annals of Surgery” (Impact factor: 10.13), on August 29th, 2020 (Japan time).
https://journals.lww.com/annalsofsurgery/Abstract/9000/Phase_II_Adjuvant_Cancer_specific_Vaccine_Therapy.94246.aspx
Overview of Research
This research utilized three types of cancer antigen peptides, that are specifically expressed in esophageal squamous cell cancer cells and are strong inducers of cancer-attacking cytotoxic T lymphocytes (CTL*2).
The 5-year survival rate of patients with advanced esophageal cancer whom underwent radical resection after preoperative chemotherapy or chemo-radiotherapy was investigated. It was shown that administration of the vaccine resulted in the prolonging of survival by approx. double (60%), compared with 32.4% without the vaccine. Furthermore, looking at the ability of the peptide-specific CTL-inducing functionality of the 3 cancer antigen peptides (figure 1), the number of CTL-inducing peptides increased, which resulted in suppression of relapses (figure 2). The survival period of esophageal cancer patients, that had confirmed induced CTL from 2 or more types of peptides, were significantly increased.
This research is not only worthy of attention as the world’s first phase 2 clinical trial(*7) to obtain a positive result for a cancer vaccine treatment, it is also considered to be a successful example of a new way to develop medicine, in the same way as new discoveries in the basic research field of immune checkpoint inhibitors (*3), but with doctors spearheading the clinical trials.
Furthermore, although funding arrangements were troublesome in the implementation of this research, for the goodwill of the many patients currently suffering, hoping for a new medicine, the patients association established by Prof. Nakamura of Tokyo University’s Institute of Medical Science, Human Genome Center, provided financial support enabling the research to continue at a facility at Kindai University.
Research Background
Squamous cell cancer, which accounts for nearly 95% of the esophageal cancers in the Japanese population, is fast-growing and easily invades the surrounding vital organs, making it easily become difficult to ablate, and as it spreads extensively and at a high frequency to lymph nodes in the early stage, and so is a disease with extremely poor prognosis. For cases of esophageal cancer where ablation is an option, except for early stage cases, although preoperative chemotherapy is the standard treatment used with the aim of eradication of systemic micro-metastases, over half of patients who survive with three or more lymph node metastases left over have about a 20% 5-year survival rate, despite the preoperative chemotherapy. Therefore, it is necessary to prevent recurrence by postoperative adjuvant therapy, but presently there is no effective treatment and therefore there is a pressing need for its development.
Furthermore, with the discovery of PD-1, and the significant developments in immune checkpoint inhibitors and clinical trials, a complementary relationship with immune checkpoint inhibitors, which neutralize the cancer cells’ ability to evade immunosurveillance, there is a great anticipation that vaccine therapy can be used to amplify CTL, which are used as a weapon to attack cancer.
About Publication
Research paper title: Phase II adjuvant cancer-specific peptide vaccine therapy for
esophageal cancer patients curatively resected after preoperative
therapy with pathologically positive nodes; possible significance of
tumor immune microenvironment in its clinical effects
Journal name: Annals of Surgery (Impact factor: 10.13)
Co-authors: Kindai University, Faculty of Medicine・・・Takuji Yasuda (lead author), Kohei Nishiki, Yoko Hiraki, Hiroaki Kato, Mitsuru Iwama, Osamu Shiraishi, Atsushi Yasuda, Masayuki Shinkai, Yutaka Kimura, Yasushi Sukegawa, Yasutaka Chiba, Motohiro Imano, Takao Sato, Hitoshi Shiozaki
Juntendo University Graduate School of Medicine, Research Support Center・・・Kazuyoshi Takeda
Tokyo University Institute of Medical Science・・・Yusuke Nakamura
Details of Research
The administered cancer antigen peptides present as antigens through the medium of human leukocyte antigen (HLA) on the surface of antigen-presenting cells. There are various types of HLA, with differing binding properties. This trial used three new cancer antigen peptides (URLC10, CDCA1, KOC1), all of which are expressed in squamous cancer cells and have a high associativity with HLA-A*2402, which is found in 60% of the Japanese population. The study was designed to target advanced esophageal cancer patients in stages 2 and 3, who were pathologically examined to have lymph node metastasis and who had had preoperative chemotherapy or chemo-radiation therapy before radical resection. Patients who were HLA-A*2402 positive were treated with the cancer-specific vaccine therapy, and those who were negative for HLA-A * 2402 were observed without any treatment until recurrence was confirmed. The administration of the cancer-specific peptide vaccine therapy treatment was started independently within 2 months of operations. The three types of cancer antigen peptides were administered subcutaneously or by intradermal means every week for the first 10 weeks and every 2 weeks for the following 10 times. Regardless of whether remissions occurred, the therapy was administered for the full 20 times. In the investigative phase 2 trial, for each group there was a target of having 30 patients, with final registered numbers being 33 for the vaccine group and 30 for the control group. The only harmful events recorded were skin reactions, from the injection of the vaccine; there were no other critical reactions. All of the patients, except three who could not attend hospital due to early stage recurrence, successfully completed the full course of treatment.
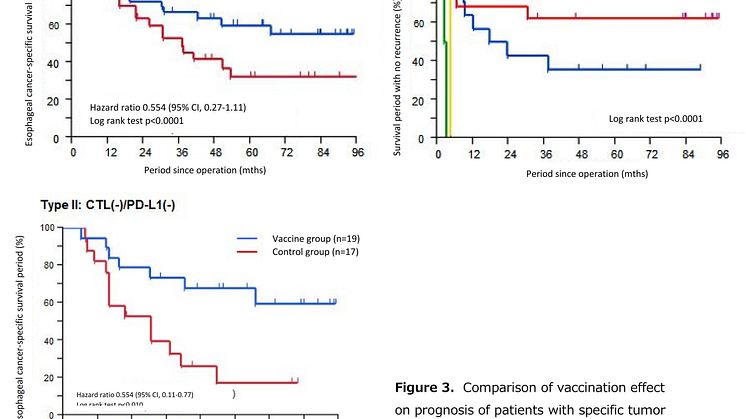
The primary evaluation item for this study, the relapse-free survival*4, showed favorable results for the vaccine group, and for the “period of survival with esophageal cancer*5”, the 5-year survival rate for the control group was 32.4%, and the vaccine group was 60%, giving a hazard ratio*6 of 0.554, p=0.045 demonstrating significant improvement of prognoses. (Figure 2) shows that as the number of cancer antigen peptides increased, the peptide-specific CTL-inducing abilities of the three types of cancer antigen peptides resulted in greater suppression of recurrences. The period of survival with esophageal cancer was clearly prolonged in cases in which CTL induction was confirmed by two or more types of the cancer antigen peptides.
Additionally, a supplementary study was conducted on whether the cancer-specific vaccine therapy was effective from the standpoint of the micro-environment of the tumor in any type of patient case. The tumor microenvironment was evaluated by using immunostaining on resected samples, with the presence or absence of CTL infiltration into the interior of the tumor and the presence or absence of PD-L1 expression, which transmits inhibitory signals to CTL, on the cancer cell surface. The results showed that, in patients with CTL(-)/PD-L1(-) tumors, which accounted for about 60% of the vaccine group, the esophageal cancer-specific 5-year survival rate was 68%, compared with 17.7% for the control group. This marks close to a 50% improvement in survival rates, with a hazard ratio of 0.31 (95% confidence interval: 0.11-0.77), p<0.010; effectiveness is clearly demonstrated. Although there were only 5 cases in figure 3 of patients who had CTL(-)/PD-L1(+) tumors, the vaccine was observed to have absolutely no effect. It is conjectured that the inhibitory signal of the PD-L1 negated the effect of the CTL induced by the vaccine. These results show with certainty that the CTLs induced contribute positively to prognosis, and also this suggests that in patients with PD-L1 expression, using cancer-specific vaccine therapy in conjunction with immune checkpoint inhibitors may be effective. It is believed this is an important step towards new developments in the individualization of immunotherapy.
Further, this research is a model case for drug discovery and an intermediary for basic research, clinical trials spearheaded by physicians and enterprise-level phase 3 clinical trials*7. Patient registration for a phase 3 clinical trial has been competed, and final analysis is scheduled for next year. It is expected efficacy equal to this study will be proven, and Japan will approve the world’s first cancer-specific vaccine therapy.
Glossary
※1 Cancer-specific peptide vaccine
Cancer cells contain specific abnormal proteins, which become antigens. These antigens produce peptide degradants, a section of which expresses on the cell surface. These serve as a marker for T cells to recognize the cell as a foreign object. The former is known as cancer-specific antigens and the latter as cancer antigen peptides. Of the cancer-specific antigens, the most easily recognisable part for T-cells are called the epitopes, and the therapy method that utilizes that part as the cancer antigen peptide is called cancer-specific peptide vaccine therapy.
(Peptides are commonly composed of 2-50 amino acids, if they contain upwards of this they are known as proteins.)
※2 CTL
Known as killer cells or cytotoxic T lymphocytes. Kills cancer cells and cells infected by viruses and so on.
※3 Immune checkpoint inhibitor
The immune system is regulated by a balance between promotion and suppression. CTLs introduced through peptide vaccine attack specific cancer cells, cancer cells express PD-L1 on the cell surface, they bind with the PD-1 molecules on the surface of the CTL cells, and evade immune attacks by transmitting a signal to inhibit the actions of the cell. This medicine blocks the signal to inhibit the CTL attack, stimulating the ability of the cell to attack, and this mechanism applies to Opdivo and Pembrolizumab, etc.
※4 Relapse-free survival
Postoperative period in which patient does not experience relapse.
※5 Period of survival with esophageal cancer
The date from surgery to death, with the cause of death being esophageal cancer.
※6 Hazard ratio
The hazard ratio is a term used in stats; a method to objectively compare the relative degree of the risks of clinical trials, etc. A hazard ratio of 1 indicates that there is no risk differential between the treatment under consideration in a clinical trial (group A) and the conventional treatment (group B). A figure under 1 determines group A as being more effective – with the smaller the figure the more effective the treatment. Figure 2 shows an example of the hazard ratio being 0.31, indicating that in patients with CTL(-)/PD-L1(-), the peptide vaccine reduced the risk of death from esophageal cancer by 69%.
※7 Phase 2 and 3 clinical trials
Clinical trials have three stages. In phase 1 tests the toxicity and dosage of a medicine. Phase 2 clinical trials evaluate the effectiveness and safety of the medicine. Phase 3 clinical trials are a large-scale randomized trials to prove the efficacy and safety of a medicine – mainly undertaken by enterprises.
For media enquiries, please contact koho@kindai.ac.jp , Public Relations Department, Kindai University.
