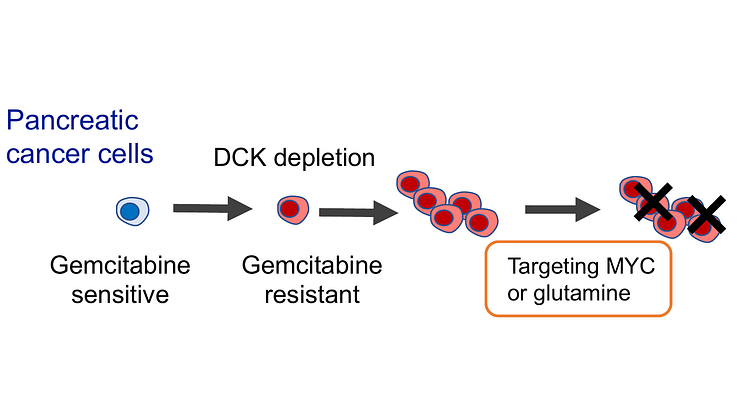
Study Reveals Novel Therapeutic Target for Severe Acute Pancreatitis Caused by Fungi -- Kindai University
Severe acute pancreatitis (SAP) is a life-threatening condition with high mortality rates. While earlier research linked gut fungi translocation to SAP, the specific mechanism was unclear. Japanese researchers have now pinpointed the protein "LRRK2" as the key trigger for SAP induced by gut fungi.