Press release -
Human FUS Protein Suppresses the Production of Toxic Repeat Polypeptides that Cause Neurodegenerative Diseases -- Kindai University
Osaka [22 August 2023] The interaction of FUS with GGGGCC repeat RNA alters the noncanonical RAN translation and suppresses amyotrophic lateral sclerosis and related conditions, find researchers
The molecular pathogenesis of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) is poorly understood. Now, Japanese researchers have discovered a key regulatory mechanism involved in C9orf72-linked ALS/FTD (C9-ALS/FTD). Their findings indicate that in C9-ALS/FTD, the human FUS protein can interact with RNAs containing GGGGCC repeats and suppress their translation by altering their three-dimensional structures, inhibiting neurodegeneration. These findings could guide therapies for currently incurable diseases such as C9-ALS/FTD and other repeat expansion disorders.
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are both highly debilitating and incurable neurodegenerative diseases with similar genetic and neuropathological characteristics. Abnormal expansions of GGGGCC repeat sequence in the noncoding region of the C9orf72 gene is the most common genetic mutation for familial ALS/FTD. In patients with C9orf72-linked ALS/FTD (C9-ALS/FTD), these expanded repeats are translated to produce neurotoxic dipeptide repeat proteins (DPRs) via a process known as noncanonical repeat-associated non-AUG (RAN) translation. Nevertheless, the regulatory machinery controlling RAN translation is not clear, hampering therapeutic interventions against these neurodegenerative disorders.
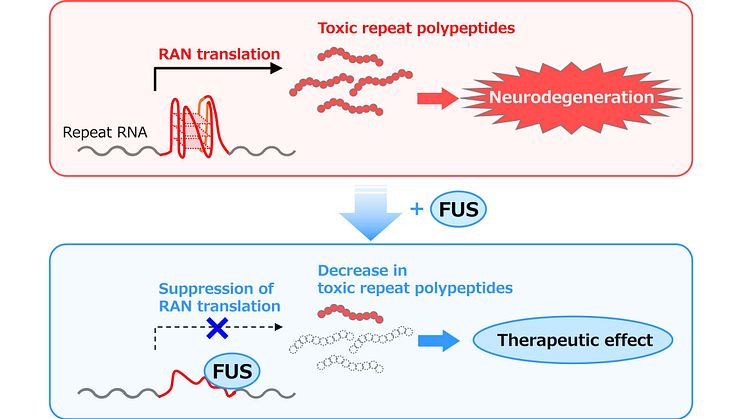
In a study published in eLife on July 18, 2023, Japanese researchers describe a previously unrecognized regulatory mechanism for RAN translation in C9-ALS/FTD. Their findings suggest that an RNA-binding protein (RBP) called FUS can interact with RNAs containing the GGGGCC repeat and block RAN translation, thereby inhibiting the production of toxic DPRs and suppressing neurodegeneration.
Professor Yoshitaka Nagai from Kindai University, the study’s lead investigator, explains, “DPRs are produced from GGGGCC repeat-containing RNAs via RAN translation. They impair several biological pathways, including nucleocytoplasmic transport and the dynamics of membrane-less organelles, and thus contribute greatly to neurodegeneration.” He adds, “This prompted us to investigate the regulatory mechanisms behind RAN translation. Specifically, we focused on the role of RBPs that target GGGGCC repeat-containing RNAs.”
Prof. Nagai’s team—which included Yuzo Fujino and Morio Ueyama from Kindai University, Hideki Mochizuki from Osaka University, Toshiki Mizuno from Kyoto Prefectural University of Medicine, Hideki Taguchi from the Tokyo Institute of Technology, and other dedicated researchers—used a drosophila (fruit fly) model to conduct their investigations. They established drosophila models of C9-ALS/FTD with the expanded GGGGCC repeats. They also confirmed that these flies had the phenotypic features—eye degeneration, motor dysfunction, and the production of DPRs. While screening for RBPs that bind to GGGGCC repeat RNA, they found that the human RBP FUS—when co-expressed in C9-ALS/FTD fly models—strongly suppressed eye degeneration and DPR production. “Although we found other RBPs that also strongly inhibited eye degeneration in the screening, FUS was the only one to do so without inhibiting the expression of GGGGCC repeat-containing RNA itself. So, we explored the protective mechanisms of FUS further,” muses Prof. Nagai.
Since FUS is an RBP, as a next step, the team used a filter binding assay to examine whether FUS interacts with GGGGCC repeat RNA in vitro. The results indicated that FUS does bind to GGGGCC repeats in a sequence-specific manner.
Interestingly, FUS is preferably bound to the 3D G-quadruplex structure of GGGGCC repeat RNA instead of its hairpin structure. Given that these 3D structures are important for RAN translation—the process that gives rise to toxic DPRs—the researchers explored the effects of FUS on the structure of GGGGCC repeat RNA. Based on circular dichroism (CD) and nuclear magnetic resonance (NMR) spectra, which are structural indicators, the researchers concluded that the G-quadruplex structure of GGGGCC RNA is considerably altered in the presence of FUS. Subsequent experiments in a cell-free in vitro translation system demonstrated that FUS directly binds to GGGGCC repeat RNA, resulting in suppression of RAN translation and DPRs production.
In conclusion, these results provide compelling evidence that FUS suppresses RAN translation and neurodegeneration in C9-ALS/FTD by directly interacting with GGGGCC repeat RNA and modifying its 3D structure. Accordingly, this study represents a major leap in our understanding of the pathogenesis of C9-ALS/FTD and other disorders caused by repeat expansions, such as spinocerebellar ataxia.
The team behind this mammoth study is excited about the prospective applications of their research. Indeed, the findings could advance the development of therapeutics for ALS, FTD, and related diseases. Interventions targeting specific polypeptides could hold the key, and perhaps these diseases will not be incurable in the near future.
Reference
Title of original paper: FUS regulates RAN translation through modulating the G-quadruplex structure of GGGGCC repeat RNA in C9orf72-linked ALS/FTD
Journal: eLife
DOI: 10.7554/eLife.84338
URL: https://doi.org/10.7554/eLife.84338
Additional information
Latest Article Publication Date: 18 July 2023
Method of Research: Experimental study
Subject of Research: Animals
Conflicts of Interest Statement: Morio Ueyama, Daisaku Ozawa, Tomoya Taminato, Toshihide Takeuchi, Yoshitaka Nagai: He previously
belonged to the Department of Neurotherapeutics, Osaka University Graduate School of Medicine, which is an endowment department supported by Nihon Medi-Physics Co., AbbVie GK., Otsuka Pharm Co., Kyowakai Med. Co., Fujiikai Med. Co., Yukioka Hosp., Osaka Gyoumeikan Hosp., Kyorin Co., and Tokuyukai Med. Co. The other authors declare that no competing interests exist.
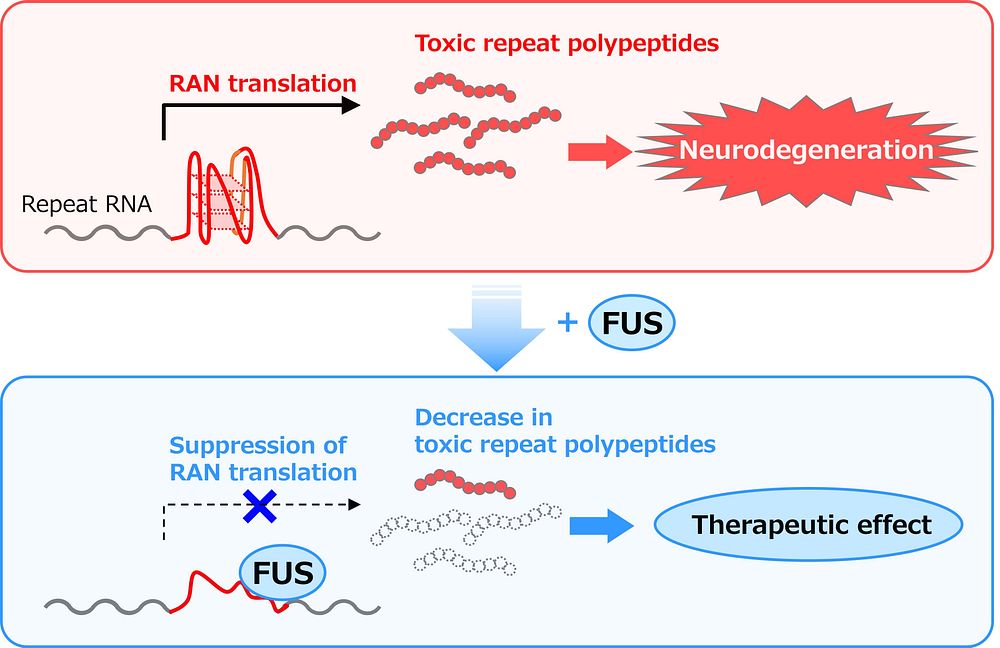
Image title: Researchers identify the molecular links between GGGGCC repeats in RNAs and neurogenerative diseases such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD).
Image caption: This study demonstrates that the human FUS protein, which has been implicated in ALS and related conditions, interacts with GGGGCC repeats in RNAs to regulate RAN translation and suppress neurodegenerative conditions in C9orf72-linked ALS/FTD.
Image credit: Dr. Yoshitaka Nagai from Kindai University
License Type: Original content
About Kindai University
Kindai University was established in 1949 after the merger of Osaka Technical College (founded in 1925) and Osaka Science and Engineering University (founded in 1943). Over the past several decades, the university has transformed into a comprehensive educational organization with an ever-growing reputation. Kindai University has over 2,200 full-time faculty members, 6 campuses, and 18 research centers. As an academic institution offering a broad range of programs from across disciplines, Kindai University strives to impart practical education while nurturing intellectual and emotional capabilities. The university’s academic programs are fully accredited by Japan’s Ministry of Education, Culture, Sports, Science and Technology as well as by the National Institution for Academic Degrees and University Evaluation.
Website: https://www.kindai.ac.jp/english
About Professor Yoshitaka Nagai from Kindai University
Prof. Yoshitaka Nagai is the Chairman of the Department of Neurology, Department of Medicine, Kindai University. He graduated from the Osaka University School of Medicine in 1990 and completed his postdoctoral fellowship at Duke University. His research focuses on the molecular pathology and therapy of diseases caused by protein misfolding as well as neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and polyglutamine disease. During his illustrious career, he has published over 140 research papers and has also been honored with an award from the Japan Dementia Society.
Funding information
This study was funded by the Ministry of Education, Culture, Sports, Science and Technology (grant nos. 17H05699, 17H05705, 20H05927, and 11013026); the Japan Society for the
Promotion of Science (grant nos. 21H02840, 20H03602, 15K09331, 19K07823, 17K07291, 17H05091, 25860733, 24659438, and 18K19515); the Ministry of Health, Labor and Welfare, Japan (grant no. H24-Soyaku-Sogo-002); the Japan Agency for Medical Research and Development (grant nos. JP15dm0107026, JP20dm0107061, JP16ek0109018, JP19ek0109222, JP20ek0109316, and JP19am0101072); and the National Center of
Neurology and Psychiatry (grants 27-7, 27-9, 30-3, 30-9, and 3-9). It also received funding from the Japan Amyotrophic Lateral Sclerosis Association IBC Grant H28, the Takeda Science
Foundation (2016 and 2017), and SENSHIN Medical Research Foundation (2018). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.



