Press release -
Next-generation prebiotic promotes selective growth of beneficial bacteria -- Paving the way for new treatments for refractory clostridioides difficile colitis -- Kindai University
The research team comprised of Shin Kurihara, Associate Professor of Department of Science and Technology on Food Safety, Faculty of Biology-Oriented Science and Technology, Kindai University (Kinokawa, Wakayama); Hiroyuki Nakai, Associate Professor of Food Science Program, Faculty of Agriculture, Department of Applied Biological Chemistry, Niigata University (Niigata, Japan); Rika Hirano, a graduate program research student (3rd year) at Ishikawa Prefectural University (Nonoichi, Ishikawa) discovered that oligosaccharide (Galactosyl-β1, 4-L-Rhamnose) selectively promotes the growth of bifidobacteria and can be a next-generation prebiotic candidate. “Prebiotic” boosts the growth of beneficial bacteria and promotes gut health. Specifically, the newly discovered combination of oligosaccharide and bifidobacteria indicated the potential for a new treatment method for C. difficile colitis.
This research was published in the “Gut Microbes”, an academic journal on intestinal bacteria and microorganisms issued by Taylor & Francis (UK), on September 23rd, 2021 (21:00, JST).
1. Key points
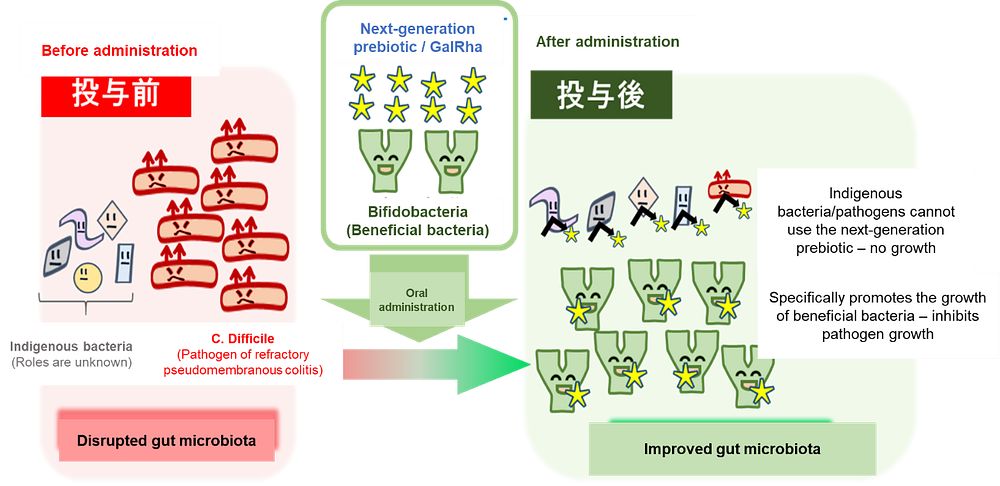
- Discovered that Galactosyl-β1, 4-L-Rhamnose (oligosaccharide) selectively promotes the growth of health-promoting bacteria such as bifidobacteria.
- Identified the gene required for the growth promotion specific to bifidobacteria, utilizing oligosaccharide as a nutrient source.
- Suppressing the growth of C. difficile by orally taking the specific type of oligosaccharide in combination with bifidobacteria.
2. Overview
Prebiotics have been taken orally in the form of supplement or food additive to improve intestinal environment. Since it needs to reach the large intestine, non-digestible sugar in the human host is generally used. However, after reaching the large intestine the prebiotic can be “taken” by indigenous bacteria, and thus sufficient amount of prebiotic is likely unavailable to the beneficial bacteria. In the course of this research, it was confirmed the possibility that many of the existing prebiotics can be utilized by various types of intestinal bacteria. As the study of intestinal bacteriology has advanced, it has been also revealed that various bacteria in the intestine have health-damaging effects (Cell, 2016, 165:842-853; Cell, 2016, 167:1339-1353.) When developing prebiotic, care must be taken to suppress the growth of pathogenic bacteria. This study aimed to develop a next-generation prebiotic that specifically promotes the growth of beneficial, health-promoting bacteria.
3. Details of the research
The research team chose 27 dominant species in the intestinal microbiota to culture in vitro along with bifidobacteria, lactic acid bacteria, and pathogenic bacteria. Among 11 types of galacto-oligosaccharides, the team successfully screened*5 Galactosyl-β1, 4-L-Rhamnose (the “GalRha”) as a prebiotic that specifically promotes the growth of bifidobacteria.
By co-culturing bifidobacteria and C. difficile, pathogens of pseudomembranous colitis, in a medium containing GalRha, it was revealed that the growth of C. difficile. was suppressed.
Aiming for clinical application, we cultured human fecal samples, bifidobacteria, and difficile, and found that the growth of C. difficile was significantly suppressed in the medium containing GalRha compared to the medium without GalRha. The result of this research indicates that combination of GalRha and bifidobacteria may lead to a new treatment for the refractory C. difficile colitis.
4. About publication
Journal name:
Gut Microbes(Impact factor: 10.245@2020)
Research paper title:
Next-generation prebiotic promotes selective growth of bifidobacteria, suppressing Clostridioides difficile
Authors:
Rika Hirano1,2 Mikiyasu Sakanaka 1, Kazuto Yoshimi 3,4, Naohisa Sugimoto 5, Syogo Eguchi 5, YukoYamauchi 3,4、Misaki Nara 1, Shingo Maeda 1, Yuta Ami 2, Aina Goto 6, Takane Katayama 1, 6, Noriho Iida 7, Tamotsu Kato 8, Hiroshi Ohno 8, Satoru Fukiya 9, Atsushi Yokota 9, Mamoru Nishimoto 10, Motomitsu Kitaoka 5,10, Hiroyuki Nakai 5*, Shin Kurihara 1,2*
*Corresponding authors
Affiliations:
1 Research Institute for Bioresources and Biotechnology, Ishikawa Prefectural University
2 Faculty of Biology-Oriented Science and Technology, Kindai University
3 Institute of Medical Science, the University of Tokyo
4 Graduate School of Medicine, Osaka Universit
5 Faculty of Agriculture, Niigata University
6 Graduate School of Biostudies, Kyoto University
7 Kanazawa University Hospital
8 Riken Center for Integrative Medical Sciences
9 Research Faculty of Agriculture, Hokkaido University
10 Institute of Food Research, National Agriculture and Food Research Organization
