Press release -
Alyref and Gabpb1 -- Gene Targets to Overcome Preimplantation Arrest in Cloned Embryos Discovered -- Kindai University
Osaka [27 September 2023] Novel insights into the genetic underpinnings of early embryonic arrest in cloned animals
Animal cloning can be used to conserve endangered animals, but its efficiency remains low due to high rates of preimplantation arrest in cloned embryos. Japanese scientists have now discovered the specific genes responsible for this phenomenon. Their findings show that the incomplete activation of the Alyref and Gabpb1 genes causes early embryonic arrest, while the supplementation of these genes supports normal embryonic growth. These findings could guide genetic manipulation strategies to optimize animal cloning.
Animal cloning, a procedure in which genetically identical copies of animals are generated, is valuable for preserving genetic materials of species, improving livestock for agriculture, and conserving endangered species. Unfortunately, the efficiency of animal cloning remains low, significantly hindering these applications. In particular, a technique called interspecies somatic cell nuclear transfer (SCNT) is required for cloning wild and endangered species. However, most embryos produced via this technique become arrested at the “preimplantation” stage -- a very early stage in embryonic development -- leading to cloning failure. The culprits responsible for preimplantation arrest have remained elusive -- until now.
In a study published on 28 August 2023 in the international journal Life Science Alliance, a team of researchers from Japan uncovered one of the programming mechanisms underlying preimplantation arrest in cloned embryos. Through a comprehensive experimental analysis, these researchers have shown that the incomplete activation of the genes Alyref and Gapbp1 leads to preimplantation arrest in cloned mouse embryos. Hailing the significance of these findings, the study’s principal investigator Professor Kei Miyamoto, from the Faculty of Biology-Oriented Science and Technology, Kindai University, Japan, elaborates, “There have been major efforts toward improving the developmental potential of cloned SCNT embryos. Our study provides mechanistic insights into the developmental arrest of these embryos, highlighting molecular reprogramming processes that occur after SCNT.” The research team also included his colleague, Mr Shunya Ihashi.
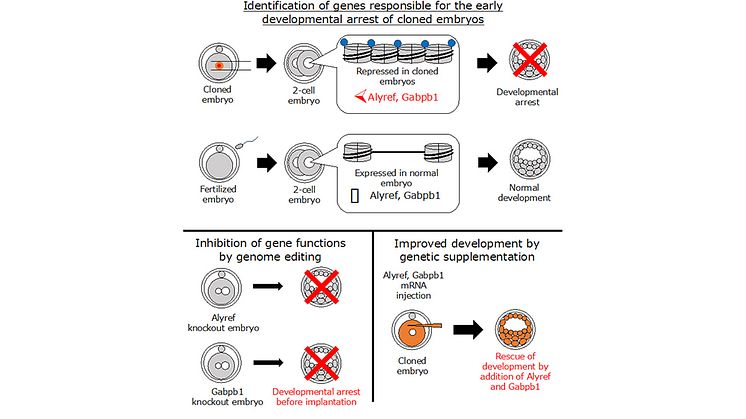
In recent years, studies have shown that a specific histone modification marker known as H3K9me3, which regulates gene expression, is associated with cloning failures. Therefore, the group led by Prof. Miyamoto focused on embryonic genes that contained this marker. A differential comparison of gene expression profiles in arrested embryos vs. normally developing embryos was performed. This list was narrowed down using siRNA-based screening experiments, leading to the identification of two key genes, Alyref and Gabpb1, as regulators of development in cloned embryos.
Subsequently, the researchers investigated how the knockdown of these genes would affect the development of fertilized embryos. Using the cutting edge CRISPR/Cas9 technique, they “deleted” the expression of Alyref and Gabpb1 from fertilized embryos at the 2-cell stage. The results were clear — embryos could not develop normally in the absence of Alyref and Gabpb1 expression. Notably, the converse of this was also true. When Alyref and Gabpb1 were artificially introduced into SCNT embryos at the 2-cell stage, the cloned embryos showed efficient preimplantation development. Hence, genetic manipulation — i.e., the “addition” of Alyref and Gabpb1 — helped overcome the problem of preimplantation arrest in cloned embryos.
“With this study, we have found one reason cloned embryos often fail to develop past the very early developmental stage,” comments Prof. Miyamoto. “These findings are significant not only because they answer a long-standing question in the field but also because they tell us how embryos are ‘reprogrammed’ after cloning,” he adds.
This study by Prof. Miyamoto and his large team of Japanese scientists marks a significant step in achieving their end goal — the conservation of endangered species. As their next step, the team seeks to improve the interspecies SCNT technique using their latest discovery. With the worsening of climate change, these developments could be instrumental in preserving the precious genetic material from several wild and endangered animals and saving them from extinction. For now, the findings from this study will help in elucidating the intricate molecular program controlling the different stages of embryonic development in cloned embryos.
Reference
Title of original paper:
Incomplete activation of Alyref and Gabpb1 leads to preimplantation arrest in cloned mouse embryos
Journal:
Life Science Alliance
DOI: 10.26508/lsa.202302296
URL: https://doi.org/10.26508/lsa.202302296
IMAGE INFORMATION
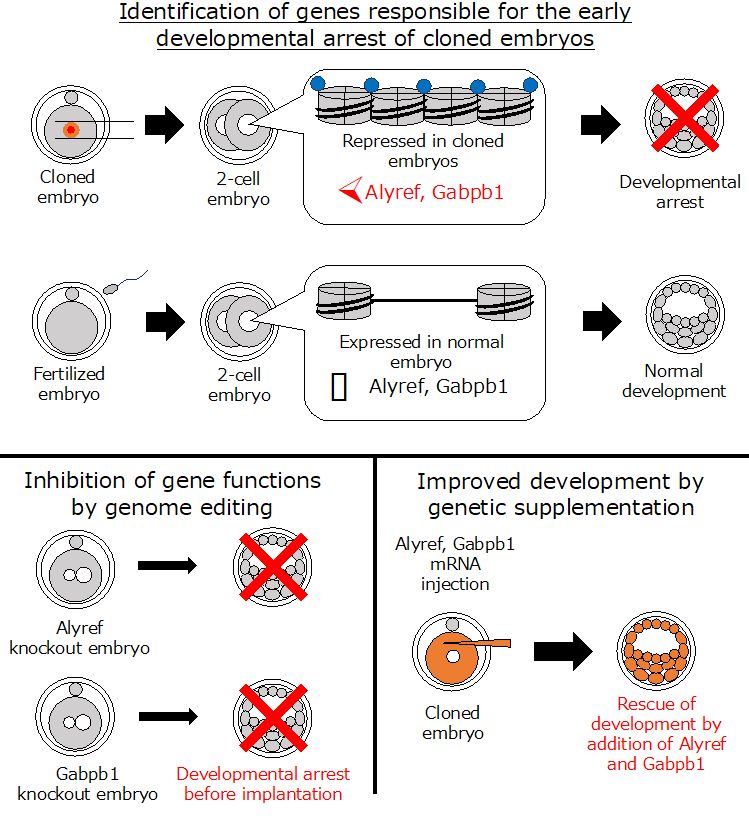
Image title:
Researchers discover the genes responsible for early developmental arrest in cloned animal embryos.
Image caption:
The incomplete activation of the genes Alyref and Gabpb1 leads to the preimplantation arrest of cloned embryos according to Japanese researchers. They further demonstrate that the supplementation of these genes can rescue embryo development and promote normal growth.
Image credit:
Kei Miyamoto from Kindai University
License Type:
Original content
About Kindai University
Kindai University was established in 1949 after the merger of Osaka Technical College (founded in 1925) and Osaka Science and Engineering University (founded in 1943). Over the past several decades, the university has transformed into a comprehensive educational organization with an ever-growing reputation. Kindai University has over 2,200 full-time faculty members, 6 campuses, and 18 research centers. As an academic institution offering a broad range of programs from across disciplines, Kindai University strives to impart practical education while nurturing intellectual and emotional capabilities. The university’s academic programs are fully accredited by Japan’s Ministry of Education, Culture, Sports, Science and Technology as well as by the National Institution for Academic Degrees and University Evaluation.
Website:
https://www.kindai.ac.jp/engli...
About Professor Kei Miyamoto from Kindai University
Dr. Kei Miyamoto is an Associate Professor in the Faculty of Biology-oriented Science and Technology, Kindai University. In 2009, he received his PhD from Kyoto University for his work on mammalian nuclear transfer and reprogramming, before embarking on his postdoctoral research career at the University of Cambridge. In 2015, he returned to Japan and started a research group at Kindai University. His research currently focuses on programming mechanisms present in eggs and oocytes that govern early embryonic development. Prof. Miyamoto has over 50 publications to his credit and is a recipient of the prestigious Japan Academy Medal.
Funding information
This study was supported by JSPS KAKENHI Grant Numbers JP19H05271, JP19H05751, JP20K21376 to K Miyamoto by The Naito Foundation, Takeda Science Foundation, a Kindai University Research Grant (19-II-1) to K Miyamoto.



